
Higher and foundation tiers
Crude oil despite being one of the world's most valuable natural resources is a smelly thick black liquid when it comes out of the ground. The main reason for this is that it is a mixture of thousands of different hydrocarbon molecules. Some of these hydrocarbon molecules are small molecules such as the gases methane, ethane and propane which are dissolved in the crude oil. There are also many medium sized hydrocarbon molecules such as those found in petrol or diesel as well as large hydrocarbon molecules that are found in bitumen, tar and greases. Now recall that hydrocarbons are compounds containing only the elements hydrogen and carbon.
There are thousands of valuable hydrocarbon molecules all mixed together in crude oil and it is the job of an oil refinery to separate out these valuable hydrocarbons from the crude oil. At an oil refinery this mixture of hydrocarbons present in the crude oil is separated into different fractions which contain similar sized molecules. This is easily done since similar sized molecules have similar boiling points.
As we mentioned above crude oil is not very useful when it first comes out of the ground because it is a mixture of many different hydrocarbon molecules. Now at an oil refinery the crude oil is separated into different useful parts called fractions. This separation takes place in a tall structure called a fractionating column and the process used to separate the crude oil into its fractions is called fractional distillation.
The image below shows a fractionating column some of the valuable hydrocarbon fractions that are obtained from crude oil and the uses these fractions are put to.
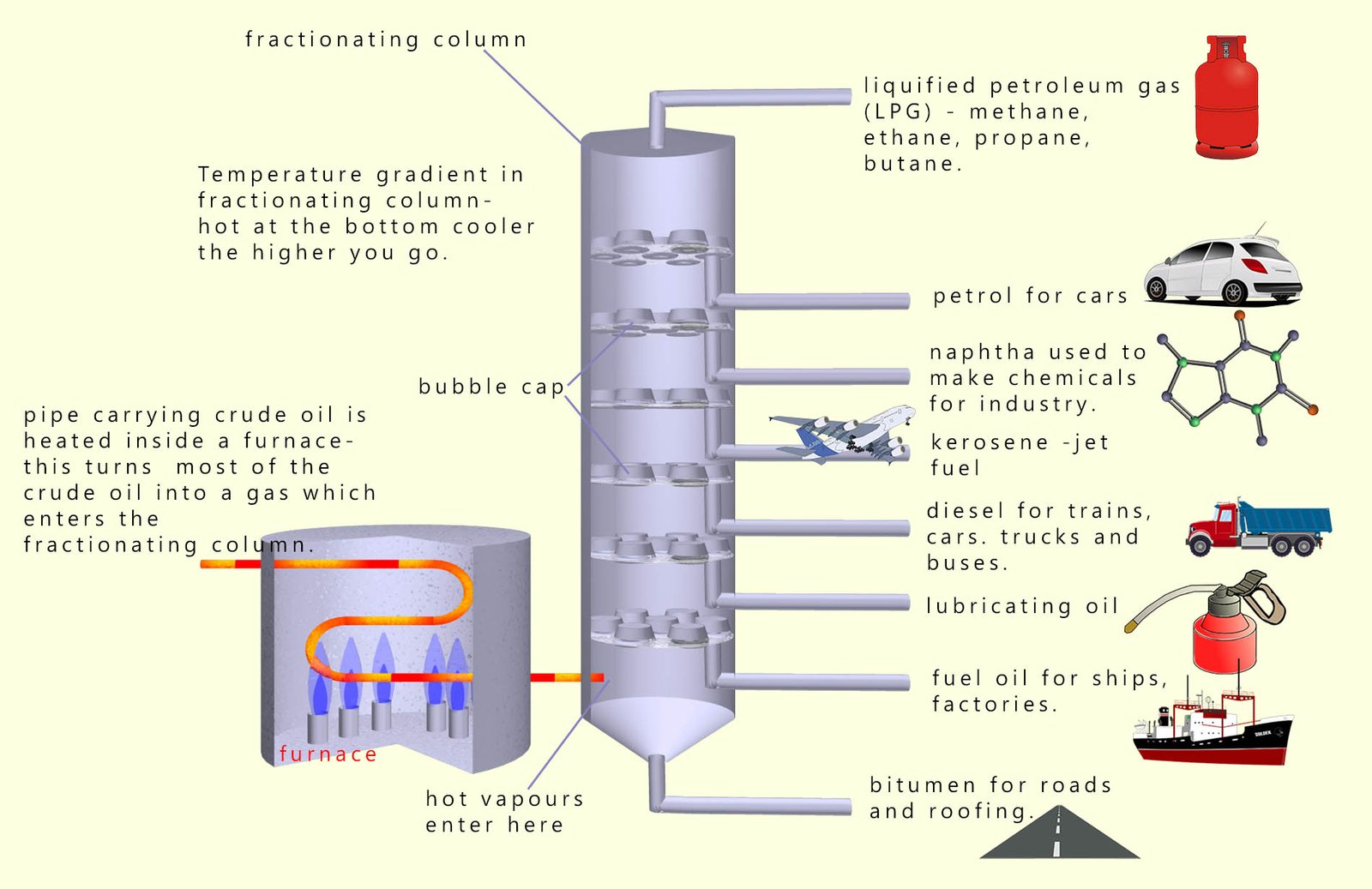
The mixture of hydrocarbons present in crude oil is separated into various fractions at an oil refinery. These fractions are themselves mixtures of many thousands of similar sized hydrocarbon molecules. Since these molecules are similar in size they have boiling points that are relatively close to each other. The crude oil is separated in a fractionating column. To separate the crude oil into its various fractions the crude oil is:
Match up the terms with their correct definitions. Simply click the term and then its correct definition. Correct responses will turn green.
The larger the hydrocarbon molecule the higher will be its boiling point, this is largely due to more and stronger intermolecular bonding between the hydrocarbon molecules. This stronger intermolecular bonding between the hydrocarbon molecules means it takes more energy to separate the molecules which means they will be less volatile and less flammable. Click here for more info on how intermolecular bonding affects the boiling points of hydrocarbons.
The fractionating column will basically separate out hydrocarbon molecules according to their boiling points into separate parts or fractions. Each fraction will contain many different hydrocarbon molecules each with similar boiling points and similar sizes.
As you can see from the image above lots of very valuable substances such as:
Try the quick activity below to review your understanding of fractional distillation:
Click where each fraction would condense in the fractionating column.
Refinery gases (very small molecules)
Kerosene
Bitumen (very large molecules)
Try the quick quiz below to review your understanding of fractional distillation:
Answer these quickly. Instant feedback is shown after each choice.
1) What property is mainly used to separate crude oil into fractions?
2) Where is the fractionating column hottest?
3) Small hydrocarbon molecules mostly condense...
4) A fraction collected from the column is best described as a...
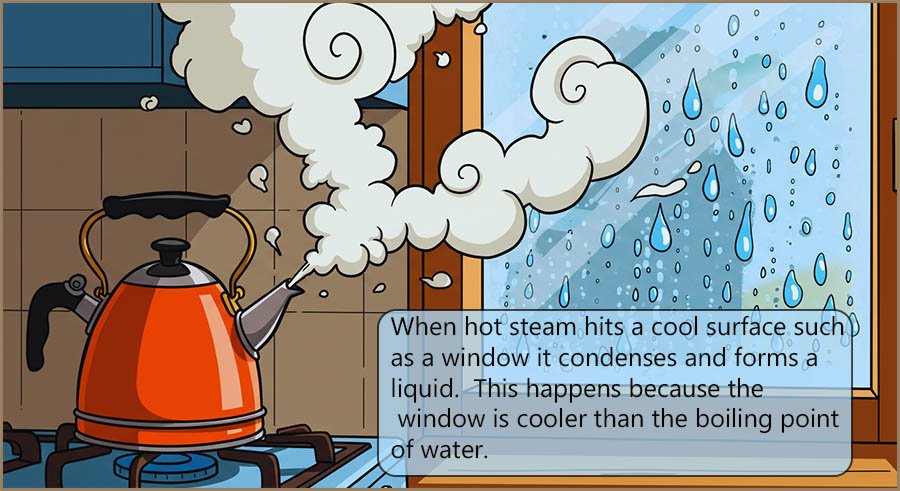
5) When vapour meets a cooler surface (below its boiling point), it will...
Complete the paragraphs below by filling in the blanks to complete the sentences summarising the main points mentioned above. The words to complete the sentences are shown in the yellow boxes below. Simply select the correct word from the drop-down menus to complete the two paragraphs.
Why not use the summary table below to make set of revision flashcards or revison notes on the fractional distillation of crude oil?
| 🧪 Fraction | 🔬 Molecule size | 🌡️ Boiling point | 📍 Where it condenses | ⚙️ Main uses |
|---|---|---|---|---|
| 💨 Refinery gases | Very small molecules | Very low | Top of column | Bottled gas, heating fuel |
| 🚗 Petrol | Small molecules | Low | Upper part of column | Fuel for cars |
| ✈️ Kerosene | Medium molecules | Medium | Middle of column | Jet fuel |
| 🚚 Diesel / gas oil | Larger molecules | Higher | Lower part of column | Diesel engines |
| 🛣️ Bitumen | Very large molecules | Very high | Bottom of column | Road surfacing, roofing |
Tick the statements you now feel confident about: